Parallel (if only portions of the liver irradiated effects are minimal as the liver can function with half or more loss of function. The liver has the ability to regenerate. Acute Side Effects: Jaundice occurs if 75% or more is irradiated (caused by increased amounts of bilirubin in the blood, which is a waste product from RBC), anorexia. Serial Vs Parallel Dilution SECOND: Also use a serial dilution when the dilution factor is so large that the amount of stock solution needed to make the dilution in one step (using the formula C 1V 1 = C 2V 2) is too small to measure accurately. Remember that the smallest volume you can measure with the micropipettors is 2 μL. The dilution factor chosen for the series of calibration standards is achievable by using serial dilution. The progression of calibration standard concentration is always a geometric series. Consider the example of making the first standard at 1/3 the concentration of the known, the next calibrant would be 1/9th the concentration of the known. In a parallel dilution the stock serves as the sole source for all the dilutions (as opposed to serial dilutions in which each dilution serves as the source for the subsequent dilution). An advantage of parallel dilutions over serial dilutions, is that errors tend to have less significance as one dilution error is not likely to affect the others. A serial dilution is a series of sequential dilutions used to reduce a dense culture of cells to a more usable concentration. The easiest method is to make a series of 1 in 10 dilutions.
Serial Dilution Vs Parallel Dilution

Click to see full answer.
Serial Dilution 1 100
Consequently, what are the advantages of serial dilution?Serial Dilution Vs Parallel Dilution
Serial dilution has many advantages: the materials necessary are typically already present in the lab and require no special engineering. Conditions can be adjusted as the experiment progresses (e.g., drug concentrations increased as drug resistance improves).
Furthermore, what is serial dilution and why is it used? Serial dilution is the stepwise dilution of a substance in solution. Serial dilutions are used to accurately create highly diluted solutions as well as solutions for experiments resulting in concentration curves with a logarithmic scale.
Secondly, is direct or serial dilution more accurate?
The direct dilution method uses far less sample than the serial dilution method. This figure shows only the first four concentrations via direct dilution. Essential to direct dilution is the ability to accurately transfer extremely small volumes of stock solution, which is generally not possible with pipets.
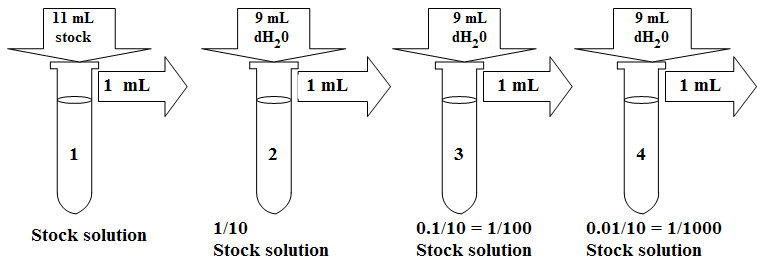
Serial Vs Parallel Dilution
What is the advantage of performing a serial dilution instead of a single dilution?
Serial Dilution Chemistry
Easier and Faster Preparation of Calibration StandardsThe errors introduced with each successive dilution drops proportionately with the solution concentration. Preparing a series of calibration standards by this method reduces the amount of required time.